PIPELINE
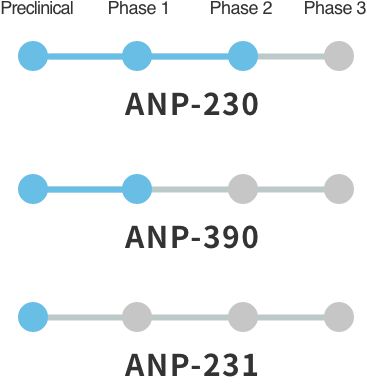
Selective voltage-gated sodium channel Nav1.7, Nav1.8, and Nav1.9 inhibitor ANP-230
Primary indication
The infantile episodic limb pain syndrome and other peripheral neuropathic pains.
Current sodium channel inhibitors for local anesthesia and developing sodium channel inhibitors have some CNS and cardiovascular-related side effects due to their lack of selectivity. We hope that our selective voltage-gated sodium channel inhibitor will have good analgesic efficacy without CNS, cardiovascular, and muscular-related safety issues and might become one of best approaches to cure the peripheral neuropathic pain, including the infantile episodic limb pain syndrome for which there are no effective drugs.
About the infantile episodic limb pain syndrome
-
Research groups in Kyoto University and Akita University found the cause for the episodic limb pain syndrome during infancy and childhood.
-They named it as the infantile episodic limb pain syndrome.- - Infantile-onset episodic limb pain (Akita University Graduate School of Medicine and Faculty of Medicine)
Selective voltage-gated sodium channel Nav1.7 inhibitor ANP-390
Primary indication
Peripheral neuropathic pain
ANP-390 is a selective Nav1.7 inhibtor, different from ANP-230. ANP-390 also has less CNS- related and CV-related side effects, which current analgesic drugs usually induce. In addition to it, it is expected that ANP-390 has efficacy for not only peripheral neuropathic pain but also peripheral itch.
Action of ANP-230
Image of transmission of pain in healthy people
Image of transmission of pain in patients with neuropathic pain
Transmission of pain in patients with neuropathic pain
When the peripheral sensory nerves are damaged, the expression level of Na+ channels in the damaged area and the dorsal root ganglion of the spinal cord is modulated, and the neuronal cells are overexcited to continuously transmit pain signals.ANP-230 is expected to reduce pain by suppressing its abnormal neuronal excitation.